Guiding you to success
Strategic Reimbursement Solutions
- Market Intelligence
- Reimbursement Strategy
- Health Economics and Outcomes Research
- Evidence Generation
Market Access Solutions
- Patient Access Services
- Reimbursement Hotline Solutions
- Clinical Trial Reimbursement Services
- Payer and Stakeholder Engagement
Our 360-degree approach to market access. PRIA’s strategists align with your commercial goals, architect a sophisticated, customized portal tailored to your technology and deploy an expert team that transforms data into action - engaging payers and stakeholders to drive real results.
Clients Served
300+
Patient Access
Programs
75+
Patients
Approved
40,000+
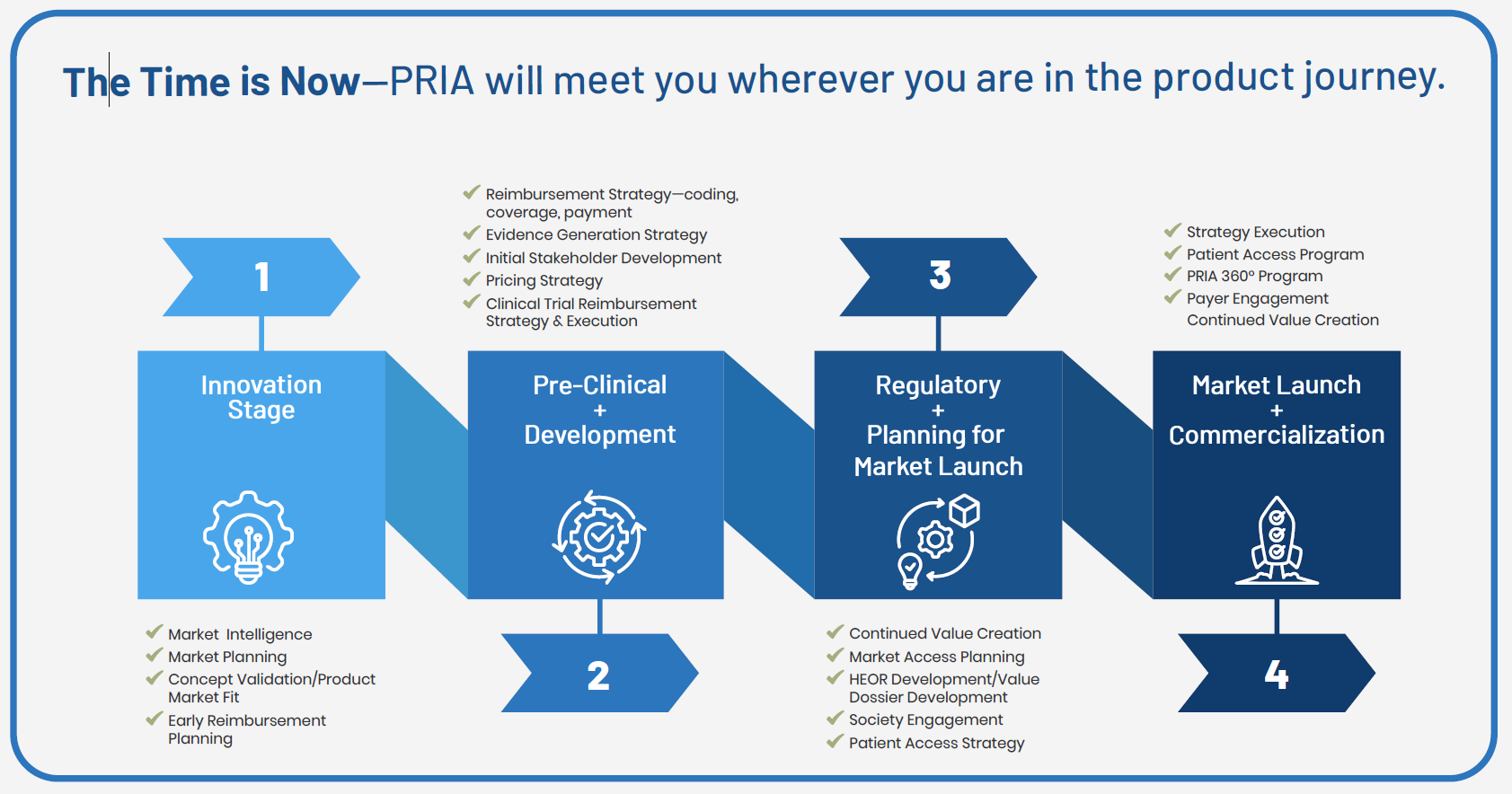
The time is now.
No matter where you are in your journey, PRIA Healthcare is your trusted partner, empowering you with expert guidance to make informed, timely decisions.
- Innovation and Discovery
- Pre-clinical and Clinical Development
- Regulatory and Commercial Planning
- Market Launch and Post Market Efforts